John Weir
Structural Biochemistry of Meiosis
Friedrich Miescher Laboratory
Faculty in: TIPP, IMPRS

Vita
- Research assistant 2004-2006 MRC-LMB, Cambridge, UK
- PhD 2006-2011 EMBL, Heidelberg and MPI Biochemistry, Martinsried, Germany
- Postdoc 2011-2016 MPI Molecular Physiology, Dortmund, Germany
- Max Planck Research Group Leader at the FML since 2017
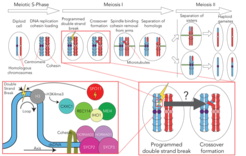
Research Interest
Meiosis is a specialised form of cell division, and the starting point of sexual reproduction, that results in the formation of haploid gametes from a diploid cell. Meiosis is biphasic; Meiosis I segregates the homologous chromosomes (one from each parent), meiosis II then separates the sister chromatids from one another. The process of meiosis is essential to maintain ploidy through the production of haploids, but also contributes to genetic diversity through the intrinsic mechanism that connects homologous chromosomes.
In order to segregate homologous chromosomes, they first must be linked. In most organisms the process starts with the cell making programme double-strand breaks in its own genome. These breaks are then repaired via homologous recombination with the homologous chromosome. Some DNA break repairs will result in crossover formation, which links the homologs in conjunction with cohesin. Double-strand break initiation and cross over formation are controlled through a dazzling array of enzymatic and structural factors. While the factors involved in these processes have been identified, there is limited understanding of the molecular mechanisms. Our lab exploits the power and flexibility of recombinant biochemical reconstitution together with hybrid structural biology, biophysics, computational biology, and genetics to provide clear and novel insights into the events in early meiosis.
Available PhD Projects
- Currently not recruiting PhD students
Selected Reading
Altmannova V, Firlej M, Müller F, Janning P, Rauleder R, Rousova D, Schäffler A, Bange T, Weir JR (2023). Biochemical characterisation of Mer3 helicase interactions and the protection of meiotic recombination intermediates. Nucleic Acids Research. 2023 May 22; 51(9):4363–4384.
Rousova D, et al. (2020). Mer2 binds directly to both nucleosomes and axial proteins as the keystone of meiotic recombination bioRχiv. July 2020. https://www.biorxiv.org/content/10.1101/2020.07.30.228908v1.
Villar-Fernández MA, et al. (2020). Biochemical and functional characterization of a meiosis-specific Pch2/ORC AAA+ assembly. Life Sci Alliance. 2020 Aug 21;3(11):e201900630. doi: 10.26508/lsa.201900630.
- Weir JR*, Klare K*, Faesen A*, et al. (2016). Insights from biochemical reconstitution into the architecture of human kinetochores. Nature 537:249-253. doi: 10.1038/nature19333.

